Bioventrix Revivent Tc System
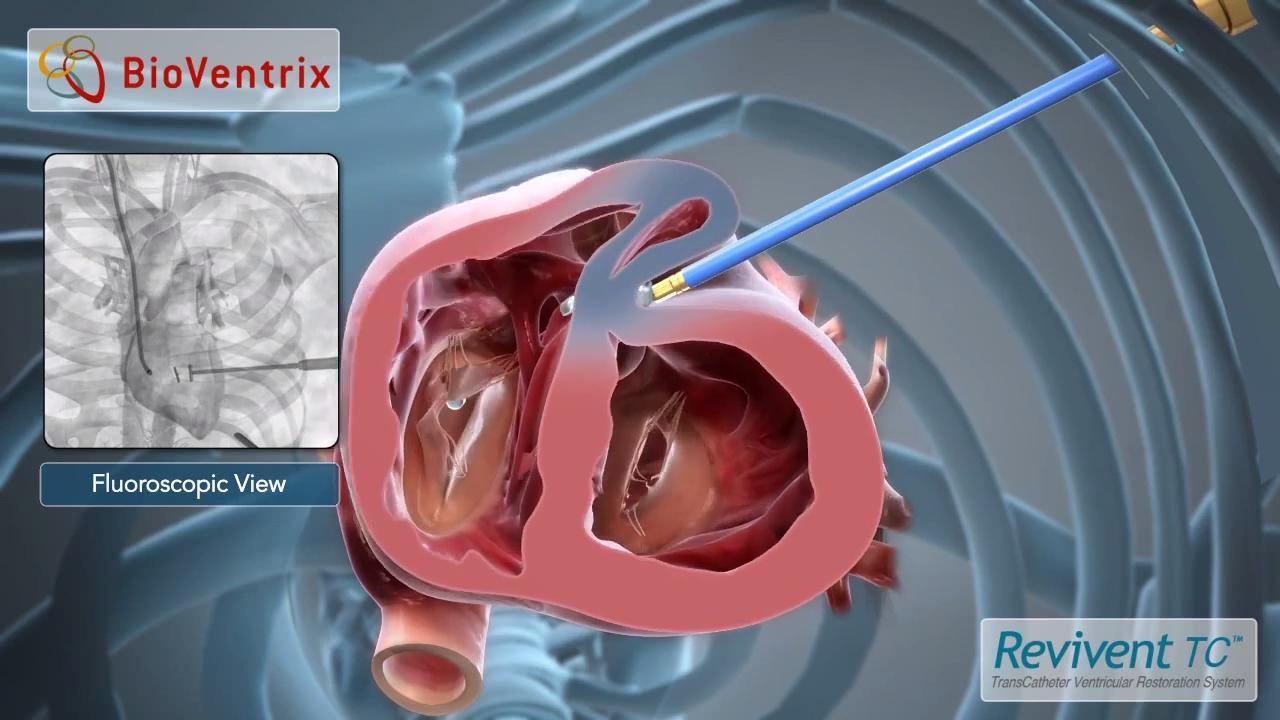
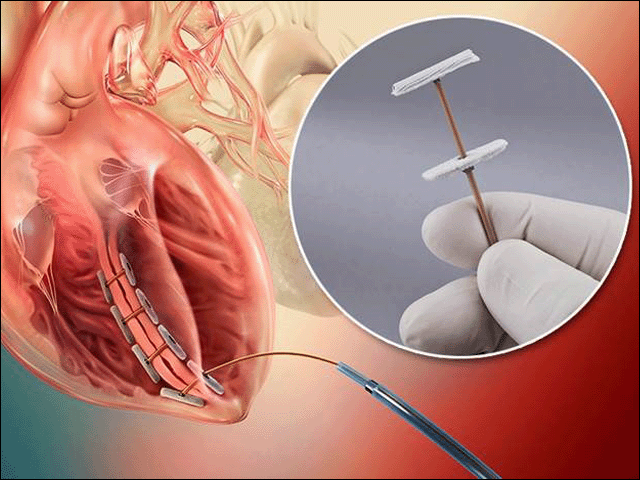
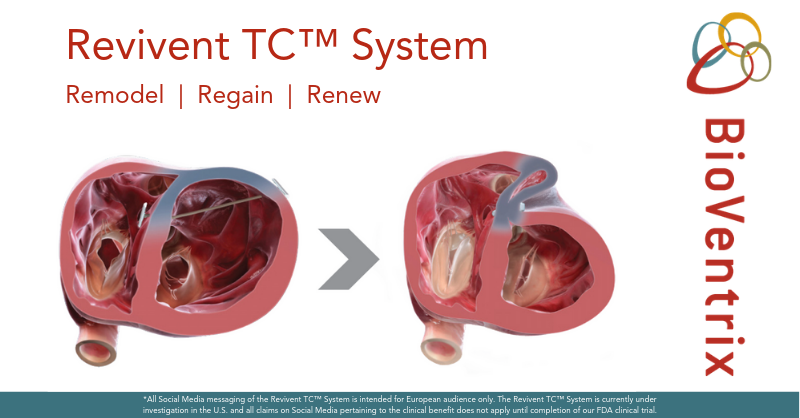
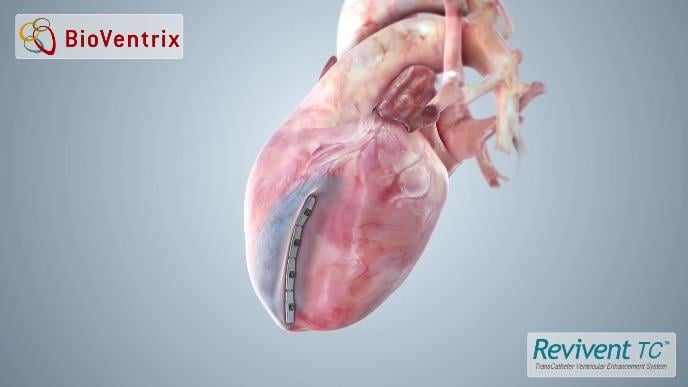
Bioventrix revivent tc system. BioVentrix is resuming the ALIVE pivotal trial of the Revivent TC Transcatheter Ventricular Enhancement System. Revivent TC System. The BioVentrix Revivent TC System offers efficacy comparable to conventional surgical ventricular reconstruction and is less invasive utilizing micro-anchor pairs to exclude scarred myocardium on the beating heart.
It is commercially available in the Europe. San Ramon CA Nov. Clinical Study of the BioVentrix Revivent TC System for Treatment of Left Ventricular Aneurysms ALIVE Actual Study Start Date.
In 2016 BioVentrix secured CE mark approval for Revivent TC system. While designed to improve quality of life and to promote reverse remodeling patients can still progress to end-stage heart failure requiring advanced therapies. Courtesy of Reaper DZ from Pixabay.
The Revivent TC System as a less-invasive therapy is a viable solution for patients who suffer from ischemic heart failure. The Revivent investigational transcatheter device uses micro-anchors to cinch together the scarred area of the left ventricle to improve heart function. The San Ramon CA-based company put the 120-patient trial on pause a few months ago because of the pandemic.
A press statement from the company details that the authors of the study. BioVentrix Revivent TC Gains FDA Breakthrough Device Status. It may provide relief for their symptoms by immediately reducing the volume of their abnormally-dilated heart and also by increasing their ejection fraction.
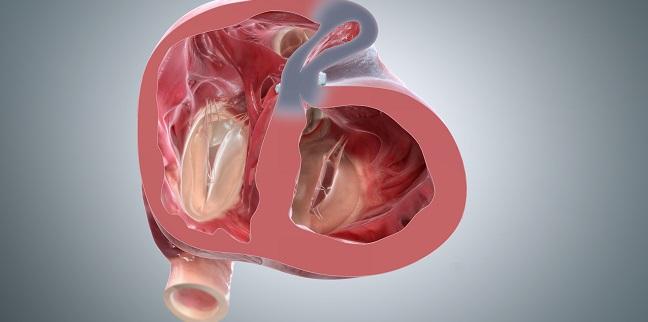
BioVentrix Inc a privately-held company with a first-in-class transcatheter-based structural heart device to treat heart failure has been granted Breakthrough Device Designation status for its Revivent TC Transcatheter Ventricular Enhancement System for heart failure. The LIVE Procedure utilizes the Revivent TC Transcatheter Ventricular Enhancement System to improve cardiac function by restoring the shape volume and resulting performance of the left ventricle LV in ischemic heart failure patients. The Revivent transcatheter ventricular enhancement system Bioventrix has been granted Breakthrough Device Designation by the US Food and Drug Administration FDA.
BioVentrix has designed REVIVE-HF study to enrol 180 patients to evaluate the treatment of ischemic cardiomyopathy induced heart failure using Revivent TC System Image. It may provide relief for their symptoms by immediately reducing the volume of their abnormally-dilated heart and also by increasing their ejection fraction.
BioVentrix Inc a privately-held company with a first-in-class transcatheter-based structural heart device to treat heart failure has been granted Breakthrough Device Designation status for its Revivent TC Transcatheter Ventricular Enhancement System for heart failure.
The Revivent TC TM System BioVentrix Inc enables a less invasive approach for left ventricular reshaping and scar exclusion in select patients with ischemic cardiomyopathy. A prospective multi-center dual-arm pivotal study of the BioVentrix Revivent TC System with 21 study vs. This study will include 126 patients of which 84 patients will be treated with the investigational device and 42 patients will be included in an active control group. BioVentrix is a privately held company specializing in the manufacturing of medical devices based in San Ramon California USA. BioVentrix Revivent TC Gains FDA Breakthrough Device Status. Outside USA English - International English The LIVE Procedure utilizes the Revivent TC Transcatheter Ventricular Enhancement System to improve cardiac function by restoring the shape volume and resulting performance of the left ventricle. BioVentrix is resuming the ALIVE pivotal trial of the Revivent TC Transcatheter Ventricular Enhancement System. Active concurrent control group allocation ratio. Estimated Primary Completion Date.
Revivent TC System is intended for treating patients with myocardial infarction. Clinical Study of the BioVentrix Revivent TC System for Treatment of Left Ventricular Aneurysms ALIVE Actual Study Start Date. The LIVE Procedure utilizes the Revivent TC Transcatheter Ventricular Enhancement System to improve cardiac function by restoring the shape volume and resulting performance of the left ventricle LV in ischemic heart failure. It may provide relief for their symptoms by immediately reducing the volume of their abnormally-dilated heart and also by increasing their ejection fraction. San Ramon CA Dec. BioVentrix is a privately held company specializing in the manufacturing of medical devices based in San Ramon California USA. BioVentrix has designed REVIVE-HF study to enrol 180 patients to evaluate the treatment of ischemic cardiomyopathy induced heart failure using Revivent TC System Image.



































Post a Comment for "Bioventrix Revivent Tc System"